GMP Antibodies for T Cell Activation
Add flexibility to your immune cell therapy manufacturing workflow with new GMP anti-CD3 and anti-CD28 antibodies. Vary the phenotypic profiles of your immune cells by optimizing CD3/CD28 antibody ratios for fine-tuned cell activation. These soluble antibodies allow for maximum flexibility to optimize protocols to your specific platform and cells.
Improve the phenotypic profiles of your T cells and reduce exhaustion by optimizing CD3/CD28 antibody ratios for fine-tuned cell activation. New GMP recombinant CD3 and CD28 antibodies allow for maximum flexibility to optimize activation protocols for your platform and cells.
Available Antibodies | |
|---|---|
GMP Antibodies | Equivalent RUO |
| GMP Anti-CD3 (OKT3) | Anti-CD3 (OKT3) |
| GMP Anti-CD28 (15e8) | Anti-CD28 (15e8) |
Equivalent RUO and GMP antibodies simplify the transition to the clinic.
Activation Unlocked
Activation Unlocked
Optimize activation to your platform and cells
Recombinantly Produced
Secure supply chain and easily scalable manufacturing
Reformatted OKT3 and 15e8 Clones
Reformatted OKT3 and 15e8 Clones
With human backbone to reduce risk associated with non-human FC receptors
Customizable Formats
Customizable Formats
Flexible fill size and format
Long Expiration Dates
Long Expiration Dates
Plan confidently with >1 year shelf life
Quality Control
Quality Control
ISO 9001, ISO 13485, enhanced QC testing
GMP CD3 & CD28 Antibodies Enable Flexible T Cell Expansion

Bio-Techne CD3 and CD28 GMP antibodies can be adapted to your protocol regardless of the starting population.
PBMCs from two independent donors were thawed and activated using CD3 and CD28 antibodies. The cells were cultured for 9-days in standard 6-well G-Rex® bioreactors using GMP T Cell Media (Cat. # CCM038-GMP) supplemented with 10 ng/mL of IL-7 (Cat. # BT-007-GMP) and IL-15 (Cat. # BT-015-GMP) cytokines. Each bar in the plots above is the average of two donors. A) PBMCs cultured with Bio-Techne soluble CD3 and CD28 antibodies have higher fold expansion compared to a competitor activator. B) Biotinylated CD3 and CD28 antibodies were conjugated with streptavidin magnetic beads and used to activate purified T cells, supporting robust expansion.
T Cell Activation and Expansion Protocols | |
Starting Cells | Antibody Protocol |
T Cells | CD3 Immobilized, CD28 Soluble |
| Bead-Conjugated | |
PBMCs | Soluble and Bead-Conjugated |
GMP Antibody Manufacturing
All Bio-Techne GMP antibodies are manufactured recombinantly, to ensure consistent performance and secure supply. Our dedicated GMP manufacturing teams strive to ensure lot-to-lot consistency and minimize supply chain roadblocks that can cause expensive manufacturing process delays.
Bio-Techne GMP Antibodies Show High Lot-to-Lot Consistency in Functional Assays
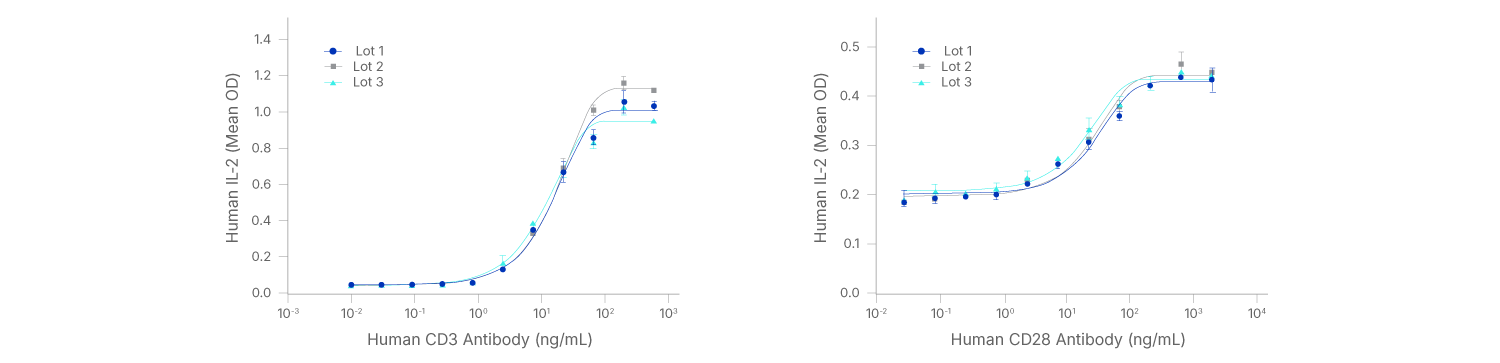
GMP CD3 and CD28 Antibodies Perform Similarly Across Multiple Lots.
Human CD3 GMP Antibody (Catalog # MAB11411-GMP) stimulates IL-2 secretion in Jurkat cells (immortalized human T cell line) treated with 5 ng/mL phorbol myristate acetate (PMA), left. The ED50 for this effect is 8.00-80.0 ng/mL. Human CD28 GMP Antibody (Catalog # MAB11412-GMP) enhances IL-2 secretion in Jurkat cells treated with 5 ng/mL PMA and 0.9 µM calcium ionomycin, right. The ED50 for this effect is 5.00-50.0 ng/mL.
Antibody-mediated stimulation occurred in a dose-dependent manner, as measured using the Quantikine Human IL-2 ELISA kit. Data from 3 separate production lots are superimposed in each figure.


